Full Text:
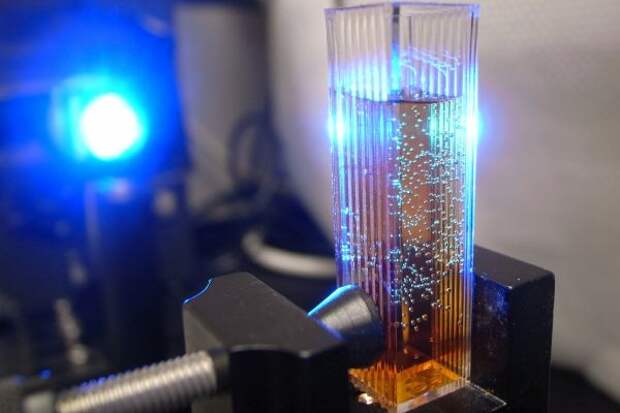
In this photo, bubbles form during water oxidation, catalyzed by a tetra-cobalt water oxidation catalyst (WOC). This catalyst, the most effective catalyst so far for water oxidation--a crucial component in generating hydrogen fuel from water, was developed by researchers in the lab of Emory University professor of chemistry Craig Hill.
The WOC research is a component of the Emory Bio-inspired Renewable Energy Center (EBREC), which aims to mimic natural processes such as photosynthesis to generate clean fuel. The long-term goal is to use sunlight to split water into oxygen and hydrogen. Hydrogen becomes the fuel. Its combustion produces the byproduct of water, which flows back into a clean, green, renewable cycle.Image credit: Laboratory of Craig L. Hill; photos courtesy of Zhuangqun "Teddy" Huang
